UC Davis Conte Center
Studying neuroimmune mechanisms of psychiatric disorders
Research
Psychiatric illnesses, including schizophrenia (SZ), affect a significant proportion of the population, yet current treatments are only partially effective for many individuals and, in the case of SZ, do little to address disabling cognitive and negative symptoms. Thus, there is a pressing need to develop biomarkers to identify at-risk individuals for early intervention and new molecular pathways to target for development of novel therapies. An increasingly compelling pathway associated with SZ is immune dysregulation. The UC Davis Conte Center brings together investigators with a unique combination and wide range of complementary expertise to address a critical gap in knowledge related to the potential links between immune dysregulation and psychiatric illness. During the previous funding period, we took a multi-pronged approach to test our Center hypothesis that early activation of the maternal immune system alters brain development in offspring leading to structural and functional changes in connectivity that are associated with the emergence of psychopathology in adolescence and young adulthood. Four important findings emerged from those studies that serve as the premise for continuing work. First, we discovered two factors in the mouse model that predict susceptibility and resilience of offspring to maternal immune activation (MIA), allowing us to study why MIA causes aberrant outcomes in only a subset of pregnancies and how it can lead to diverse phenotypes in offspring. Second, we found signatures of abnormal brain development in our male MIA NHP offspring as early as 6 months of age, indicating that the early postnatal period is critical for understanding the impact of MIA on brain development. Third, combined results from NHP and mouse models point to cortico-striatal circuitry as central to behavioral outcomes in MIA offspring. Finally, convergence between MIA NHP imaging findings and recent onset SZ support the clinical relevance of the MIA models. Moving forward, we will continue to test our original Center hypothesis across species (mouse and NHP MIA models and humans with SZ), through three specific aims:
- Identify immune signaling pathways in females before and during pregnancy that confer susceptibility or resilience to distinct subsets of MIA-induced behavioral phenotypes in offspring,
- Determine the contribution of cortico-striatal circuits to susceptibility, resilience and phenotypic heterogeneity in MIA mouse and NHP offspring and in individuals with SZ, and
- Determine how sex contributes to susceptibility, resilience and phenotypic heterogeneity in MIA offspring and individuals with SZ.
Successful completion of these Aims, which could only be accomplished in a highly integrated interdisciplinary Center as proposed, will identify causal molecular pathways in specific neural circuits critical for guiding the development of interventions optimized for the developmental age and sex of at-risk offspring following MIA. They will also reveal new immune signaling pathways that can be targeted for the development of biomarkers to identify at-risk pregnancies, and a new class of much-needed therapeutic interventions to prevent SZ and other NDDs.
- Project 1: Maternal Immunity in MIA susceptibility
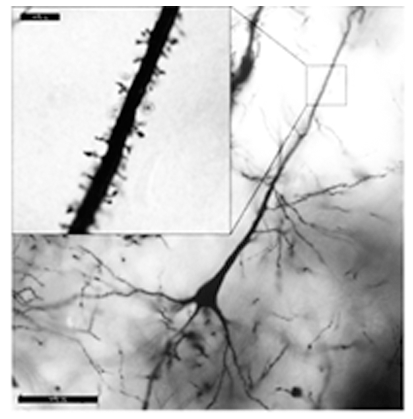 Maternal infection increases susceptibility of offspring to psychiatric and neurodevelopmental disorders, including schizophrenia (SZ).
Animal models of maternal immune activation (MIA) support this link, because mid-gestational injection of poly(I:C) induces behavioral and neuropathological abnormalities in adult offspring in domains similar to those affected in SZ.
Thus, the poly(I:C) mouse model provides an opportunity to identify the molecular and cellular underpinnings of susceptibility to MIA, which could lead to earlier diagnosis and treatment of brain disease in humans.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease:
(i) Most pregnancies are resilient to maternal infection, and
(ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently discovered a way to study both of these issues in the MIA mouse model.
Results to date have revealed — for the first time — an intrinsic factor, baseline immunoreactivity (BIR) of female mice before pregnancy, that, together with the poly(I:C) dose used to induce MIA, predicts resilience as well susceptibility to neuropathology and aberrant behaviors in offspring.
These discoveries provide a unique opportunity to identify immune signatures before and during pregnancy that underlie susceptibility and resilience to MIA, which can be used as biomarkers for susceptible pregnancies.
The central goal of this project is to identify the immune signaling pathways in females before and during pregnancy that confer susceptibility or resilience to distinct subsets of MIA-induced behavioral endophenotypes in offspring.
We will address three specific aims:
Maternal infection increases susceptibility of offspring to psychiatric and neurodevelopmental disorders, including schizophrenia (SZ).
Animal models of maternal immune activation (MIA) support this link, because mid-gestational injection of poly(I:C) induces behavioral and neuropathological abnormalities in adult offspring in domains similar to those affected in SZ.
Thus, the poly(I:C) mouse model provides an opportunity to identify the molecular and cellular underpinnings of susceptibility to MIA, which could lead to earlier diagnosis and treatment of brain disease in humans.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease:
(i) Most pregnancies are resilient to maternal infection, and
(ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently discovered a way to study both of these issues in the MIA mouse model.
Results to date have revealed — for the first time — an intrinsic factor, baseline immunoreactivity (BIR) of female mice before pregnancy, that, together with the poly(I:C) dose used to induce MIA, predicts resilience as well susceptibility to neuropathology and aberrant behaviors in offspring.
These discoveries provide a unique opportunity to identify immune signatures before and during pregnancy that underlie susceptibility and resilience to MIA, which can be used as biomarkers for susceptible pregnancies.
The central goal of this project is to identify the immune signaling pathways in females before and during pregnancy that confer susceptibility or resilience to distinct subsets of MIA-induced behavioral endophenotypes in offspring.
We will address three specific aims:
- Identify immune biomarkers and signaling pathways that underlie the range of BIR in female mice before pregnancy and that correlate with susceptibility and resilience to MIA in offspring,
- identify how the progression of the maternal gestational immune response in the mouse model dictates susceptibility or resilience to the effects of MIA in offspring, and
- determine if BIR before pregnancy, and the maternal gestational immune response, correlate with susceptibility or resilience to MIA in the non-human primate model.
Project 1 directly addresses the central hypothesis and all three aims of this Conte Center in a mechanistic manner by identifying immune signaling pathways in females before and during pregnancy that confer susceptibility or resilience to distinct subsets of MIA-induced neurodevelopmental and behavioral phenotypes in offspring. Results from this project are also essential to the success of the other projects in the Center in revealing the immune signaling pathways that drive susceptibility and resilience to sex-dependent changes in neural circuits (Projects 2, 4 and 5) and neurodevelopmental and behavioral outcomes of offspring (Projects 2-5). Working closely with Project 2, we will also test causality of two immune pathways for MIA-induced changes in striatal dopaminergic (DA) release and behaviors, developing a pipeline for testing immune pathways (Projects 1, 4) across models (Projects 2, 3, and 5) for their ability to ameliorate the effects of MIA, thereby enhancing the translational relevance of our Center.
- Project 2: Striatal circuits in MIA phenotypic heterogeneity
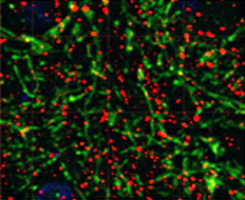 Maternal infection increases susceptibility of offspring to psychiatric and neurodevelopmental disorders, including schizophrenia (SZ).
Animal models of maternal immune activation (MIA) support this link, because mid-gestational injection of poly(I:C) induces behavioral and neuropathological abnormalities in adult offspring in domains similar to those affected in SZ.
In particular, deficits in executive function, reward processing, and dopaminergic (DA) input to striatal circuits are altered in SZ and in MIA offspring.
Thus, the poly(I:C) mouse model provides an opportunity to identify molecular targets in specific neural circuits related to SZ that could lead to earlier diagnosis and treatment of brain disease in humans.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal infection and (ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently discovered a way to study both of these issues in the MIA mouse model.
Results to date have revealed — for the first time — an intrinsic factor, baseline immunoreactivity (BIR) of female mice before pregnancy, that, together with the poly(I:C) dose used to induce MIA, predicts resilience as well as susceptibility to specific combinations of striatal-dependent behaviors and changes in immune proteins in the striatum in offspring.
The central goals of this project are to identify the changes in striatal circuits and immune molecules in offspring and the changes in cytokine signaling in the dam that confer resilience or susceptibility to specific combinations of MIA-induced behavioral outcomes.
To that end, we will address three specific aims:
Maternal infection increases susceptibility of offspring to psychiatric and neurodevelopmental disorders, including schizophrenia (SZ).
Animal models of maternal immune activation (MIA) support this link, because mid-gestational injection of poly(I:C) induces behavioral and neuropathological abnormalities in adult offspring in domains similar to those affected in SZ.
In particular, deficits in executive function, reward processing, and dopaminergic (DA) input to striatal circuits are altered in SZ and in MIA offspring.
Thus, the poly(I:C) mouse model provides an opportunity to identify molecular targets in specific neural circuits related to SZ that could lead to earlier diagnosis and treatment of brain disease in humans.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal infection and (ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently discovered a way to study both of these issues in the MIA mouse model.
Results to date have revealed — for the first time — an intrinsic factor, baseline immunoreactivity (BIR) of female mice before pregnancy, that, together with the poly(I:C) dose used to induce MIA, predicts resilience as well as susceptibility to specific combinations of striatal-dependent behaviors and changes in immune proteins in the striatum in offspring.
The central goals of this project are to identify the changes in striatal circuits and immune molecules in offspring and the changes in cytokine signaling in the dam that confer resilience or susceptibility to specific combinations of MIA-induced behavioral outcomes.
To that end, we will address three specific aims:
- Characterize behavioral changes across multiple domains in male and female MIA offspring from susceptible and resilient groups, defined by BIR of the dam before pregnancy;
- determine whether MIA alters striatal DA release and how D1- and D2-specific pathways shape striatal-dependent behaviors in susceptible and resilient male and female offspring; and
- determine whether the balance of pro-inflammatory and regulatory maternal cytokines dictate susceptibility and resilience to MIA-induced changes in cortico-striatal-dependent behaviors, DA release, and immune proteins in male and female offspring.
Our project directly addresses the main Center hypothesis, and all three Center aims, in a mechanistic manner by defining changes in cortico-striatal circuits that underlie susceptibility and resilience to MIA and by comparing phenotypes between male and female offspring. Results from this project will provide a phenotypic read-out for the maternal immune and the neurodevelopmental molecular pathways identified in Projects 1 and 4, as well as circuit-based and behavioral information in the mouse for comparison to nonhuman primate MIA offspring (Project 3), and in humans with SZ, and for the computational framework that will bridge the species (Project 5). Ultimately, this project may identify neural circuit components that can be targeted for interventions to prevent offspring from developing circuit and behavioral abnormalities in regions and domains similar to those affected in humans with SZ.
- Project 3: Neurodevelopment in an NHP MIA model
 Epidemiological studies have implicated maternal infection in the etiology of psychiatric and neurodevelopmental disorders, such as schizophrenia (SZ).
Animal models of maternal immune activation (MIA) further support the link by demonstrating that experimental activation of the maternal immune system induces changes in offspring brain and behavioral development in domains relevant to human neurodevelopmental disease.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal infection and (ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently extended the results of the rodent MIA model into a species more closely related to humans — the rhesus monkey.
Compared with rodents, nonhuman primates (NHPs) are more similar to humans in placental structure and physiology, gestational timelines, brain development, immune ontogeny, neuroanatomical organization, and behavioral complexity.
The NHP thus provides a translationally relevant model system to systematically examine issues related to MIA risk, resilience and phenotypic heterogeneity.
Results to date indicate that MIA-exposed male monkeys exhibit immune alterations in the early postnatal period, followed by reductions in frontal grey matter as early as 6 months of age and subtle impairments in social behavior and cognitive processing that emerge prior to 18 months of age.
These early developmental changes in MIA-exposed NHPs provide an opportunity to identify translationally relevant factors that predict susceptibility or resilience to prenatal immune challenge, and to explore for the first time, the impact of MIA in female NHP offspring.
We propose three specific aims:
Epidemiological studies have implicated maternal infection in the etiology of psychiatric and neurodevelopmental disorders, such as schizophrenia (SZ).
Animal models of maternal immune activation (MIA) further support the link by demonstrating that experimental activation of the maternal immune system induces changes in offspring brain and behavioral development in domains relevant to human neurodevelopmental disease.
However, critical gaps in knowledge persist related to two of the most important aspects of this risk factor for human disease: (i) most pregnancies are resilient to maternal infection and (ii) susceptible pregnancies lead to multiple distinct disorders in offspring.
We have recently extended the results of the rodent MIA model into a species more closely related to humans — the rhesus monkey.
Compared with rodents, nonhuman primates (NHPs) are more similar to humans in placental structure and physiology, gestational timelines, brain development, immune ontogeny, neuroanatomical organization, and behavioral complexity.
The NHP thus provides a translationally relevant model system to systematically examine issues related to MIA risk, resilience and phenotypic heterogeneity.
Results to date indicate that MIA-exposed male monkeys exhibit immune alterations in the early postnatal period, followed by reductions in frontal grey matter as early as 6 months of age and subtle impairments in social behavior and cognitive processing that emerge prior to 18 months of age.
These early developmental changes in MIA-exposed NHPs provide an opportunity to identify translationally relevant factors that predict susceptibility or resilience to prenatal immune challenge, and to explore for the first time, the impact of MIA in female NHP offspring.
We propose three specific aims:
- Quantify the acute maternal-placental-fetal response to prenatal immune challenge and determine the relationship with subsequent changes in NHP neurobehavioral development;
- determine the impact of MIA on species-typical social and cognitive developmental milestones in male and female NHP offspring; and
- characterize long-lasting changes in dynamic cellular immune function, peripheral inflammatory markers, and brain cytokines in NHP offspring.
Our project directly addresses the central hypothesis and all three aims of this Conte Center and leverages the unique features of the NHP model to bridge the gap between rodent MIA models and patient populations. Results from this project will provide unprecedented insight into MIA-induced changes in the primate maternal-placental-fetal environment in collaboration with Project 1. Our comprehensive assessment of NHP behavior is translationally aligned with Project 2 rodent studies and Project 5 human studies, resulting in an overarching computational framework that will bridge the species. The same NHPs that undergo behavioral phenotyping will participate in longitudinal neuroimaging (Project 5) followed by characterization of brain cytokines and changes in gene expression (Project 4). If successful, our project will identify translational biomarkers to predict which pregnancies are most vulnerable, thus providing a means to mitigate the deleterious effects of MIA during pregnancy.
- Project 4: Refining transcriptional networks in MIA
Despite substantial advances in genetics, there is a lack of understanding of how environmental or fetal-maternal factors influence neuropsychiatric disease susceptibility. Over the last four years we have shown that maternal immune activation (MIA), a risk factor for neurodevelopmental disorders, results in long-lasting regional changes in gene expression in the brain of mice and non-human primates (NHP). One set of key observations has been that variability in the maternal immune response, driven by baseline differences in immunoreactivity (BIR), likely contributes to the variability in offspring outcomes. These observations provide us with the opportunity to mechanistically connect maternal immune factors with subsequent resilience or susceptibility in changes in brain and behavior in offspring. By transcriptomic profiling of immune cells from the mothers before and during pregnancy following MIA, as well as brain cells from MIA and control offspring, at the bulk tissue and single cell level, this project provides a molecular and cellular framework for understanding the basis for differential outcomes in offspring caused by MIA across species. Specifically, we will distinguish how differential cortico-striatal gene expression relates to BIR before pregnancy and to changes in maternal immune responses during MIA. First, in collaboration with Project 3 we will characterize changes in gene expression in specific cell types in dorsolateral prefrontal cortex (PFC) and striatum in NHP MIA offspring using bulk RNAseq and nucSeq to measure transcriptome changes in cortical neurons, striatal neurons, and glial populations, including males and females (controls and MIA) from mothers with a comprehensive assessment of their immune response and fetal-placental development. Second, our team will characterize and integrate the transcriptional signature caused by MIA to identify changes in gene expression in specific cell types in the PFC and striatum of susceptible and resilient mouse MIA offspring characterized in Project 2. Third, these data will be combined with transcriptomic markers in blood from susceptible and resilient female mice and NHPs before and during pregnancy from Project 1, to identify changes in gene expression in specific immune cell types associated with MIA and variability in BIR. Fourth, we will integrate molecular data with outcomes and maternal premorbid immune response to identify biomarkers and mechanistic models of susceptibility and resilience in mothers and offspring. We will identify changes associated with maternal parameters including in blood immune cells (Aim 3), cytokine profiles (Project 1) and maternal sickness (Projects 2 and 3), and associate them with offspring response (brain cytokines, behavior: Projects 1, 2, 3; imaging changes in humans: Project 5). These data will be used to inform mechanistic models that link neuroimmune responses to molecular pathways and specific cellular responses, to the emergence of altered brain structure and behavior. The single platform, cross-species design will enable us to more definitively determine the relationship of these changes to neuropsychiatric disease in humans.
- Project 5: Systems and circuits in MIA and schizophrenia
- Longitudinal imaging studies of sex effects on phenotypic heterogeneity in MIA NHPs,
- Computational model–based analysis of frontal-striatal circuitry associated with motivation and cognitive control in SZ, and
- Mechanisms underlying sex-related phenotypic heterogeneity in SZ.
- Biostatistics Core
The overall goal of the Biostatistics Core of the UC Davis Conte Center is to ensure that sound experimental design and rigorous statistical analyses are used to address the scientific aims of the Center and advance our understanding of how early maternal immune activation alters brain development in offspring, leading to structural and functional changes in the brain that are associated with the emergence of psychopathology in adolescence and young adulthood. The Biostatistics Core is highly integrated with all components of the Conte Center and provides Conte Center investigators with comprehensive statistical support, from the conception and design of the study, the implementation of quality control methods, and the development and use of analysis strategies that will achieve estimation and hypothesis testing objectives, to the completion of data analysis and presentation of results. Core personnel consults with Center investigators throughout all phases of their projects from planning to interpretation of results. Core faculty also have a strong commitment to advancing the education mission of the Center and participate extensively in training and mentoring of Center trainees and young investigators in the areas of biostatistics and research design. Finally, faculty involved in the Core develop new biostatistical methodology as challenges and opportunities arise in pursuit of the Center's research goals.
Conte center investigators and trainees can submit an online request for statistical support.
- Administrative Support Core
The Administrative Core will provide administrative services to the UC Davis Conte Center, composed of five projects and a Biostatistics Core, promoting strong fiscal management and oversight, and effective coordination among the research groups as they pursue their interconnected and interdependent research projects. The Administrative Core will ensure optimal communication across the Center and coordinate the strategic communication of research results, as they become available, both within the Center as well as externally to the scientific and broader community. As the public face of the Center, the Administrative Core will be responsible for reporting progress to NIMH, as well as disseminating results through a diverse set of mechanisms to the scientific community, other stakeholders, and the public at large. The Core will also be responsible for the oversight of the training activities of the Center, working in coordination with the broad range of pre- and post-doctoral training programs in neuroscience and related areas at UC Davis. The Administrative Core will also oversee the evaluation of the Center's research, education, and outreach activities. In order to meet these goals, we will pursue the following five specific aims: (i) Create an organizational structure to expedite and coordinate research and provide financial and administrative oversight, (ii) Foster internal Center communication and collaboration, (iii) Train the next generation of translational neuroscientists, (iv) Engage the scientific community, local schools, mental health clinicians and the public at large through a combination of targeted activities, and (v) Measure the impact of Center research and dissemination activities.
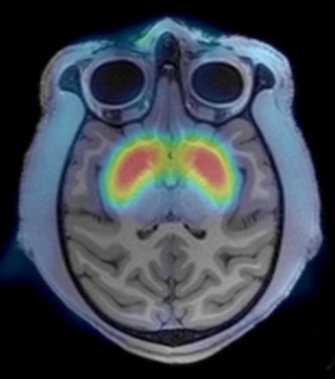 Evidence from epidemiology implicates gestational maternal immune activation (MIA) in the pathophysiology of schizophrenia (SZ).
These data, together with recent advances in our understanding of the role of immune molecules in normal brain development, have led to the overarching hypothesis of this Conte Center: that early activation of the maternal immune system alters brain development in offspring leading to structural and functional changes in connectivity that are associated with the emergence of psychopathology in adolescence and young adulthood.
Our initial pilot studies of a unique nonhuman primate (NHP) MIA model found evidence of altered social, repetitive, and self-injurious behaviors that emerged during adolescence and were associated with increased striatal dopamine (DA).
During the previous funding cycle, we found decreases in frontal cortical volumes and increases in extracellular free water (FW), a putative measure of neuroinflammation, measured using diffusion weighted MRI (DWI), in cingulate cortex gray matter (GM) in male MIA NHPs.
These changes in the developing NHP brain were present as early as 6 months postnatally, prior to the emergence of any behavioral abnormalities, and highlight the importance of the early postnatal period for understanding the effects of MIA on brain development.
In a parallel study in patients with SZ, we showed increased GM FW with maximal differences present in anterior cingulate, supporting the clinical relevance of the NHP MIA model.
In the next round of study, we focus on the effects of MIA in the early postnatal period and on brain development in female NHPs, scanning both males and females at 6 and 18 months of age.
To obtain an integrated cross-species understanding of the effects of MIA and SZ on the development of cortico-striatal functional circuitry, we will pursue the following three aims:
Evidence from epidemiology implicates gestational maternal immune activation (MIA) in the pathophysiology of schizophrenia (SZ).
These data, together with recent advances in our understanding of the role of immune molecules in normal brain development, have led to the overarching hypothesis of this Conte Center: that early activation of the maternal immune system alters brain development in offspring leading to structural and functional changes in connectivity that are associated with the emergence of psychopathology in adolescence and young adulthood.
Our initial pilot studies of a unique nonhuman primate (NHP) MIA model found evidence of altered social, repetitive, and self-injurious behaviors that emerged during adolescence and were associated with increased striatal dopamine (DA).
During the previous funding cycle, we found decreases in frontal cortical volumes and increases in extracellular free water (FW), a putative measure of neuroinflammation, measured using diffusion weighted MRI (DWI), in cingulate cortex gray matter (GM) in male MIA NHPs.
These changes in the developing NHP brain were present as early as 6 months postnatally, prior to the emergence of any behavioral abnormalities, and highlight the importance of the early postnatal period for understanding the effects of MIA on brain development.
In a parallel study in patients with SZ, we showed increased GM FW with maximal differences present in anterior cingulate, supporting the clinical relevance of the NHP MIA model.
In the next round of study, we focus on the effects of MIA in the early postnatal period and on brain development in female NHPs, scanning both males and females at 6 and 18 months of age.
To obtain an integrated cross-species understanding of the effects of MIA and SZ on the development of cortico-striatal functional circuitry, we will pursue the following three aims:
Interpretation of the results of these imaging studies will be enhanced by tissue analyses in the NHPs (Project 4) at age 18 months and measurement of the BIR and immunoreactivity of the mothers (Project 1) of the offspring we plan to image. Finally, in addition to the above study in MIA NHPs, and in line with our Center's expanded focus on MIA effects and of SZ on cortico-striatal circuitry, we will use a novel computational model–based fMRI analyses to dissect the neural circuitry underlying motivation and cognitive control deficits as well as their relationship to cortical FW and midbrain neuromelanin (NM), a novel proxy for hyperdopaminergic activity in SZ. This computational model will allow cross-species comparisons of the role for cortico-striatal circuitry in similar behavioral assays in the mouse MIA model. Finally, as in the NHP study, we will also examine the role of sex in the phenotypic heterogeneity in humans with SZ at the neural systems level and in relationship to symptoms and clinical outcomes.